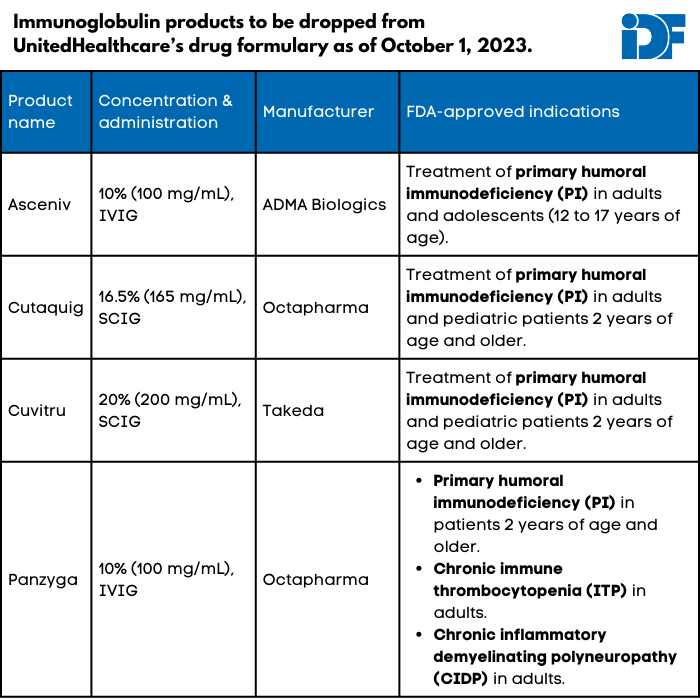
UnitedHealthcare (UHC), a private insurance company that provides health insurance to more than 25 million Americans nationwide, will be dropping four immunoglobulin (Ig) products from its formulary on October 1, 2023: Asceniv, Cuvitru, Cutaquig, and Panzyga. These formulary changes will affect individual UnitedHealthcare plans (i.e., those obtained through a marketplace) except in Massachusetts, Nevada, and New York.
It is unclear if employer-based, Medicare, or Medicaid UHC plans are affected. UHC has not responded to questions seeking clarification.
None of the affected products has a biosimilar (generic version of a biologic) or interchangeable product recognized by the U.S. Food and Drug Administration. UHC’s notice of the change declares, “Asceniv, Cutaquig, Cuvitru, and Panzyga are not medically necessary for the treatment of any diagnosis addressed within this policy,” including primary immunodeficiency (PI), Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy (CIDP), and many others. UHC did not respond to questions about how these specific products were found to be not medically necessary despite their lack of biosimilars.
IDF and GBS|CIDP Foundation International, a global nonprofit organization supporting individuals and their families affected by GBS, CIDP, and related conditions, are jointly committed to serving the individuals affected by this policy. As we work with UHC and policymakers to reverse this decision, there are steps you can take if you will be impacted.
- Talk to your prescribing healthcare provider as soon as possible to choose a ‘backup’ Ig product should you lose access to one of the products listed above.
- Be prepared to file an appeal. If you have been prescribed one of the products above and have been on it stably for a while, you may be able to successfully appeal a coverage denial to UHC.
- Check out our step-by-step guide to filing an appeal.
- In particular, have your healthcare provider provide a short letter/documentation outlining why you are on your particular product, including any history of tolerability issues with similar products.
- Let us know if you are impacted. We rely on information and stories from you to put a face on decisions that negatively impact patients.
- If you use one of these products to treat a primary immunodeficiency, share your story through Ask IDF, and IDF will be in contact for additional details.
- If you use one of these products to treat GBS, CIDP, or MMN, contact the GBS|CIDP Foundation International.
Originally published by IDF (Immune Deficiency Foundation) on September 14th, 2023
