For US Audience Only
A Study to Evaluate Safety, Tolerability, Pharmacometrics, and Efficacy of DNTH103 in Adults
with Multifocal Motor Neuropathy (MOMENTUM)
What is this trial about?
The MOMENTUM study is testing a new medicine called DNTH103 to see if it can help people with
MMN (Multifocal Motor Neuropathy).
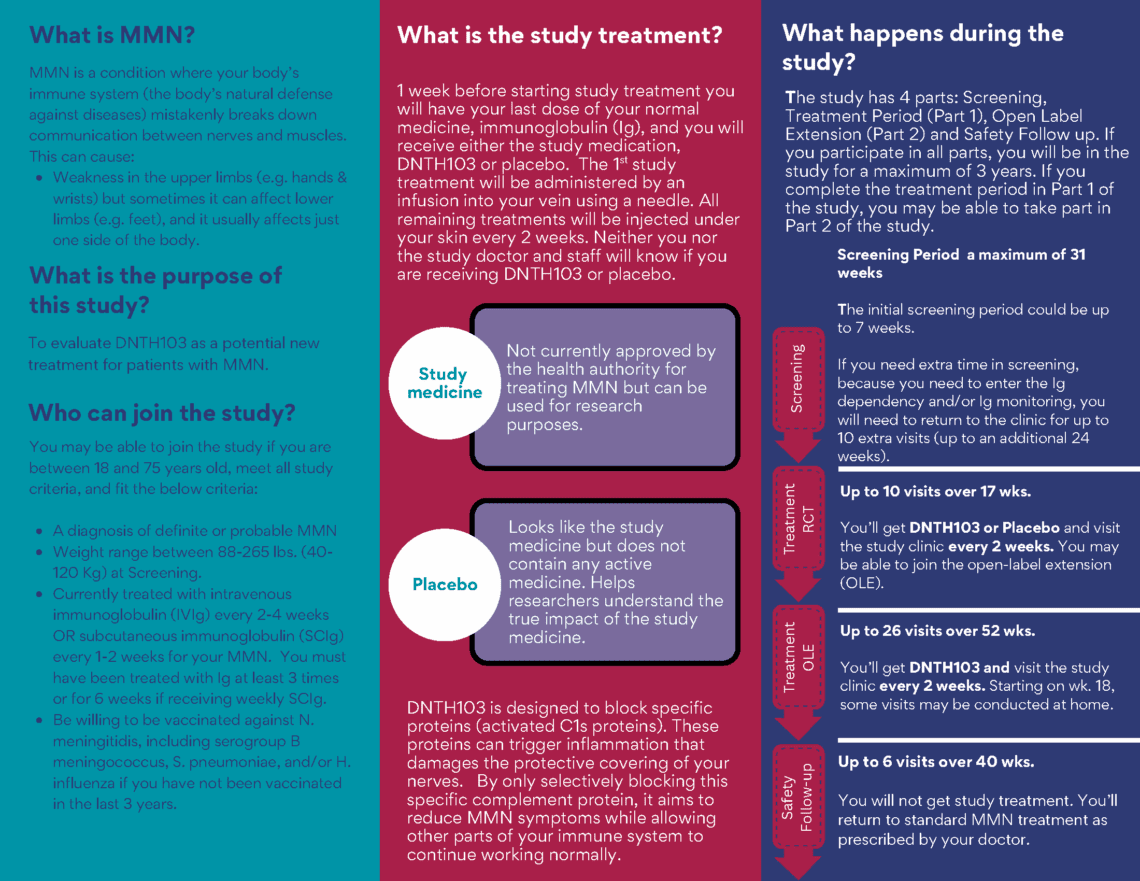
What is MMN?
This can cause:
- Weaknesses in the upper limbs (e.g. hands & wrists) but something can aƯect lower limbs
(e.g. feet). - Muscle cramping and twitching.
- Tiredness that aƯects daily activity
- In later stages, affected muscles may waste away.
About the new medicine being tested
The new medicine (DNTH103) works by blocking a specific protein (activated C1s) in your body that
may be causing nerve damage. By only blocking activated C1s proteins, DNTH103 aims to reduce
nerve damage while still allowing other parts of your immune system to fight infections.
How you’ll receive the medicine:
- First time: injected into your vein (like an IV)
- After that: As an injection under your skin every 2 weeks
How the trial works – Step by Step
- Screening Period (a maximum of 31 weeks): To check if you qualify, the study doctor will
do some tests. The initial screening period could be up to 7 weeks. If you need extra time in
screening to check if you respond to Ig treatment you will need to return to the clinic for up
to 10 extra visits (up to an additional 24 weeks.). You will receive vaccinations against N.
meningitidis, including serogroup B meningococcus, S. pneumoniae, and/or H. influenza if
you have not been vaccinated in the last 3 years. - Treatment period (up to 17 weeks): You’ll receive either the new medicine or a placebo (a
look-alike with no active medicine). One third of participants will receive placebo. - Optional extension (up to 52 weeks): Everyone receives the new medicine
- Follow-up period (up to 40 weeks): Regular check-ups after treatment end
Can I join this trial?
You might be able to join if you:
- Are between 18 and 75 years old
- Have a diagnosis of definite or probable with MMN
- Weight range between 88-265 lbs. (40-120 Kg) at Screening.
- Currently treated with intravenous immunoglobulin (IVIg) every 2-4 weeks OR
subcutaneous immunoglobulin (SCIg) every 1-2 weeks for your MMN. You must have been
treated with Ig at least 3 times or for 6 weeks if receiving weekly SCIg.
What happens during study visits?
- What happens during study visits?
- Most visits take about 3 hours
- You’ll have:
o Blood tests
o Physical check-ups
o Tests to measure your nerve and muscle function and strength
o Questions about how you’re feeling
Where allowed by local regulations:
- Transportation help may be available
- Some visits might be done at your home
Is there any cost to join?
All study-related medical care is provided at no cost to you, including:
- Study-related exams
- Study medication
MOMENTUM_GBS CIDP Foundation Website Posting_US_v1.0_08Aug2025 - IVIg during Screening period
- Vaccines
- Study-related procedures
No insurance is required to participate
Where regulations permit
- Transportation support and reimbursement may be available for patients and caregivers
- Some visits may be conducted at home
Want to learn more?
If you’re interested in learning more about the MOMENTUM trial, please email us at
clinicaltrials@dianthustx.com or speak with your healthcare provider about whether this trial might
be right for you.
More trial information can also be found at https://www.clinicaltrials.gov/study/NCT06537999