A Phase 2 clinical study in adult patients with Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Sanofi is currently recruiting patients to evaluate effectiveness and safety of a new investigational medicine called “SAR445088” for the treatment of CIDP. SAR445088 is a monoclonal antibody with a new mechanism of action as it targets the complement system directly.
All the patients participating in the study will receive SAR445088. This is a so-called “open label study”, meaning that no placebo will be used in the study.
About the investigational medicine and its mechanism of action:
SAR445088 is a humanized, second-generation IgG4 monoclonal antibody.
Antibodies are proteins created by your own body as a natural reaction to certain antigens (other proteins that are the target of the antibodies). Antigens can be foreign bodies, or in the case of autoimmune diseases, the body’s own cells or tissues. Humanized monoclonal antibodies can bind to specific antigens in the body to prevent unwanted interactions.
SAR445088 works by blocking the action of part of the complement system, which is a component of the immune system. In some diseases, complement proteins can cause destruction of cells or other tissues in the body. There is evidence supporting that the complement system may play a role in the destruction of peripheral nerve that occurs in CIDP. Given this mechanism of action, SAR445088 may be an effective treatment for diseases where the complement pathway attacks normal tissues and cells, like CIDP.
About the study:
- Number of patients
The study plans to include 90 patients in about 30 sites across North America, Europe, and Asia. The study is currently open for recruitment.
- Purpose
The purpose of the study is to evaluate if SAR445088 works to improve symptoms in three populations of adults who have CIDP:
- Participants who are currently receiving standard of care (SOC) treatment, defined as immunoglobulins or corticosteroids (50 patients)
- Participants who were treated previously with SOC treatments but without meaningful improvement (20 patients)
- Participants who have not been treated with SOC treatment (20 patients)
Additional purposes of the study are to find out how safe and tolerable the drug is, to measure the amount of study drug in your blood, and to determine if your body develops antibodies against the drug.
- Duration of the study
The screening period (including exams, tests, vaccinations) will determine if you are eligible to enter in the study. During this screening period you will be asked to receive several vaccinations. This screening period can last a maximum of 6 weeks.
Then, the study consists of two parts:
Part A: a 24-week treatment period.
Part B: an optional extension of the study after part A with a 52-week treatment period.
The diagram below illustrates the different periods of this study:
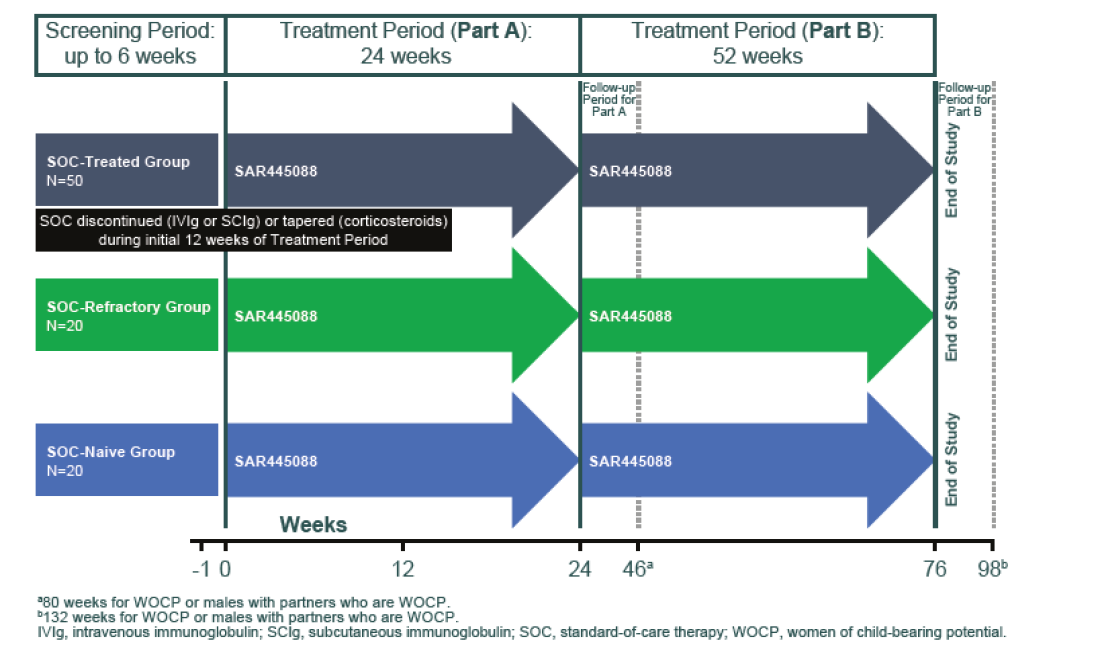
For more information on this study, please click on the direct link of this study of clinical trial website: https://clinicaltrials.gov/ct2/show/NCT04658472

Comments are closed.